einstein (São Paulo). 28/Apr/2025;23:eAO1124.
Omission of dexamethasone in prophylaxis for highly emetogenic chemotherapy in patients with breast cancer
DOI: 10.31744/einstein_journal/2025AO1124
Highlights
■ Evaluation of a corticosteroid-free antiemetic regimen.
■ Primary endpoint: 46% nausea control.
■ Secondary endpoint: 68% emesis control.
■ Comparable to standard four-drug protocols.
ABSTRACT
Objective:
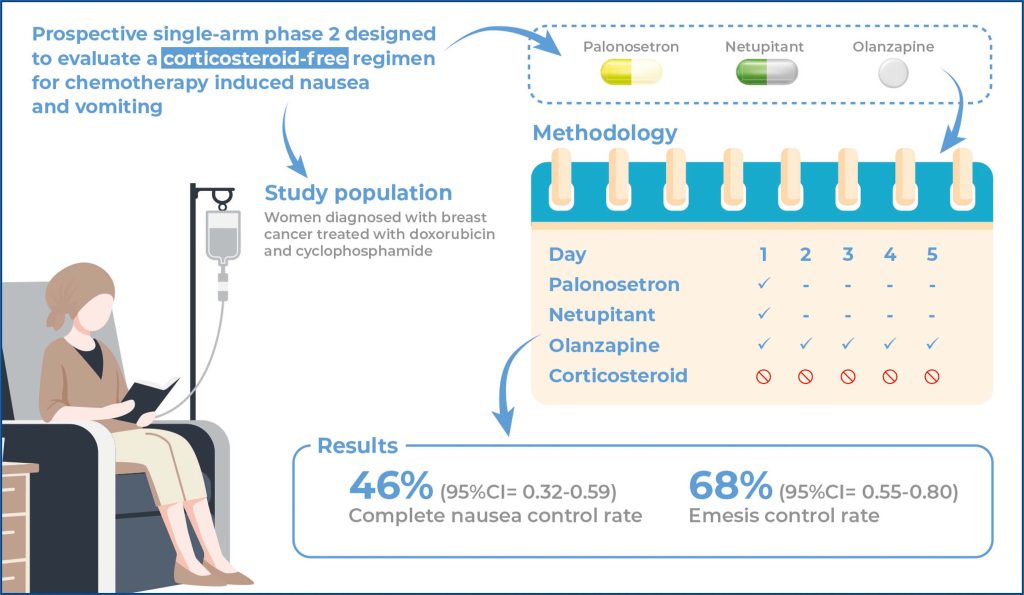
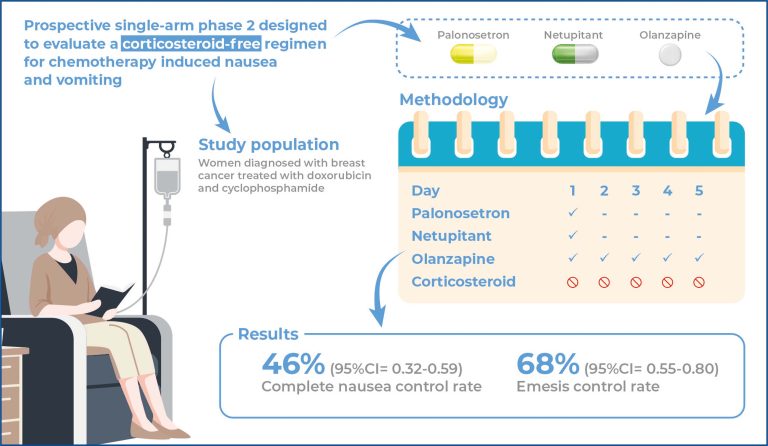
Chemotherapy-induced nausea and vomiting are highly prevalent adverse events that can lead to poor treatment adherence and a decreased quality of life. To the best of our knowledge, the complete omission of dexamethasone from any regimen for preventing nausea and vomiting has not yet been evaluated. This study aimed to evaluate the efficacy of a three-drug protocol without corticosteroids for preventing nausea and vomiting.
Methods:
This prospective, singlearm, phase II study was designed to evaluate the efficacy of olanzapine, netupitant, and palonosetron in controlling nausea and vomiting induced by emetogenic chemotherapy. Patients were assigned to receive olanzapine on days 1-5 and netupitant and palonosetron on day 1. No corticosteroids were administered. The primary endpoint was complete nausea control during the first 5 days after chemotherapy. Secondary endpoints included complete emesis control (no emesis and no use of rescue medication) and overall complete control (no emesis, no rescue medication, and no nausea).
Results:
The complete nausea control rate was 46% (95% confidence interval [95%CI] 0.32-0.59). The emesis control rate was 68% (95%CI= 0.55-0.80), and the overall control rate was 46% (95%CI= 0.32-0.59).
Conclusion:
These findings suggest thatomitting dexamethasone in highly emetogenic chemotherapy is feasible and results in nausea and vomiting control rates similar to those of the standard four-drug protocol. However, randomizedcontrolled trials are required to confirm this hypothesis.
[…]
196