einstein (São Paulo). 17/Oct/2024;22:eAO0821.
Laboratory profiles of patients hospitalized with COVID-19 pneumonia treated with tofacitinib or placebo: a post hoc analysis from the STOP-COVID trial
DOI: 10.31744/einstein_journal/2024AO0821
Highlights
Tofacitinib use did not result in meaningful changes in hematological parameters.
Tofacitinib use did not lead to clinically meaningful changes in liver enzymes.
ABSTRACT
Objective:
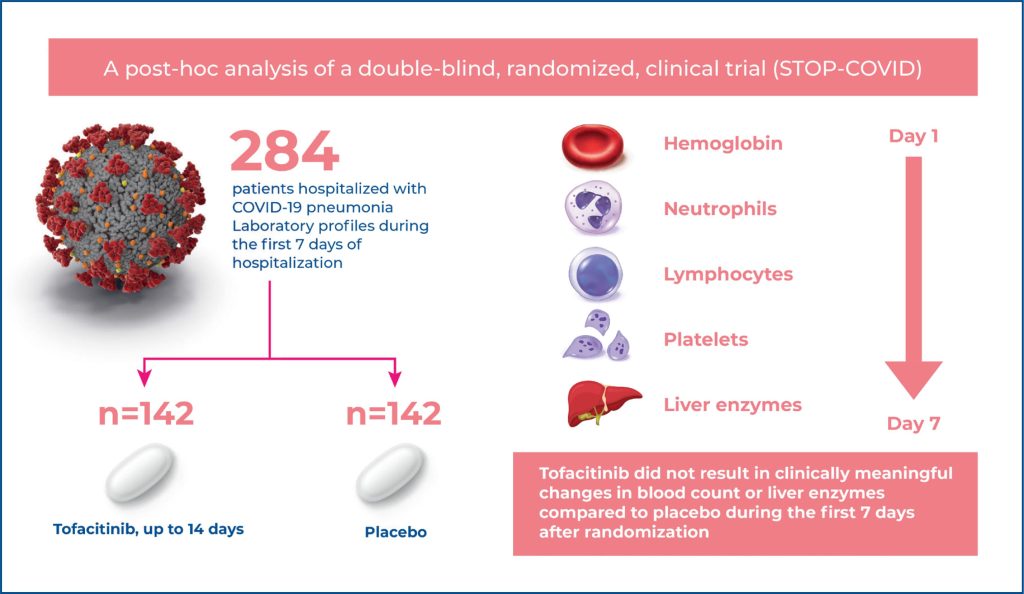
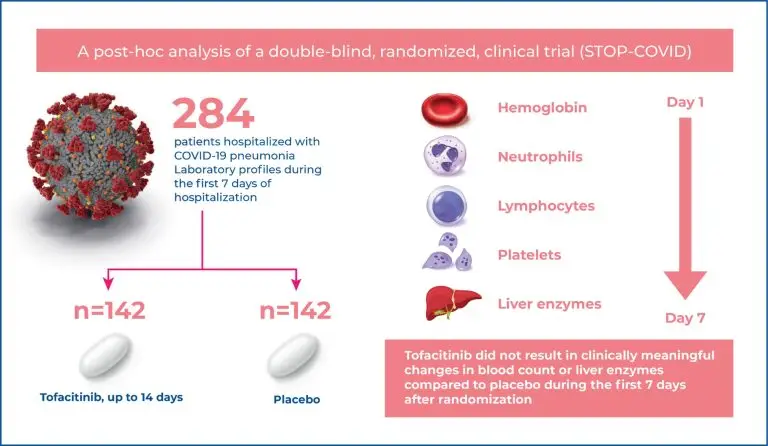
Tofacitinib, an oral Janus kinase inhibitor, has been tested against a placebo in 289 patients with COVID-19 pneumonia. We analyzed the data from the tofacitinib- and placebotreated patient cohorts to evaluate the laboratory profiles between baseline and day 7.
Methods:
We performed post hoc analyses on the following laboratory tests over time during the first 7 days after randomization: hemoglobin, leukocytes, neutrophils, lymphocytes, platelets, alanine aminotransferase, and aspartate aminotransferase.
Results:
Through the first 7 days after randomization, the levels of hemoglobin, white blood cells, neutrophils, and platelet counts were not significantly different between patients treated with tofacitinib or a placebo (all p>0.05). Nonsignificant differences were observed in aspartate aminotransferase levels over time between treatment groups, whereas alanine aminotransferase levels (U/L) were higher among tofacitinibtreated patients compared to placebo-treated patients (mean ratio, 1.30 [95% confidence interval (95%CI) = 1.14–1.48; p<0.01)].
Conclusion:
In patients with COVID-19 pneumonia, the use of tofacitinib compared to placebo did not result in clinically meaningful changes in blood counts or liver enzymes over the first 7 days after randomization.
[…]
116