2 results
10/Mar/2025
10/Mar/2025
DOI: 10.31744/einstein_journal/2025AO0964
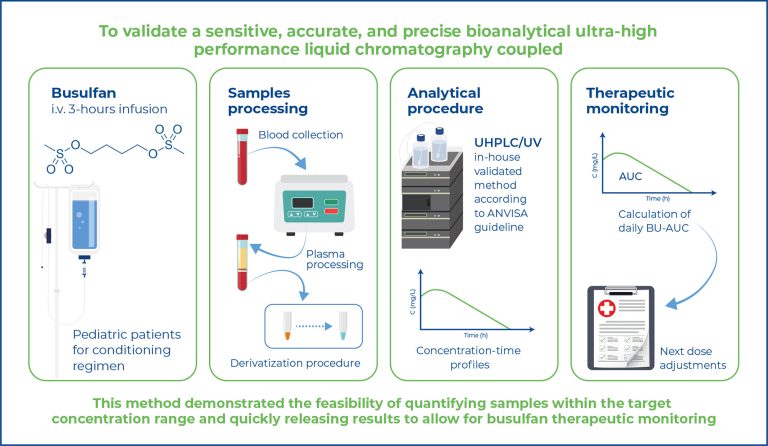
Highlights ■ We validated the UHPLC/UV method for accurate busulfan quantification in plasma. ■ Inaccuracy and imprecision were below 15%, ensuring reliable therapeutic drug monitoring results. ■ This enables effective pharmacokinetic studies with rapid turnaround times in patient samples. ABSTRACT Objective: This study aimed to validate a sensitive, accurate, and precise bioanalytical ultrahigh- performance liquid chromatography coupled with ultraviolet (UHPLC/UV) method for the determination of busulfan in human plasma using 1,6-bis-(methanesulfonyloxy) hexane as an internal standard for therapeutic drug monitoring. […]
Keywords: Busulfan; Calibration; Chromatography, high pressure liquid; Drug monitoring; Hematopoietic stem cell transplantation; Hospital, public; Pharmacokinetics
06/Feb/2019
DOI: 10.31744/einstein_journal/2019AO4396
ABSTRACT Objective Analyze the microbiological effectiveness, based on the pharmacokinetics/pharmacodynamics correlation of vancomycin in pediatric patients, and to propose dose adjustment. Methods This is an observational, cross-sectional study, conducted in a pediatric hospital, over a 1-year period (2016 to 2017). Children of both sexes, aged 2 to 12 years, were included in the study; burn children, and children in renal replacement therapy were excluded. For the pharmacokinetic analysis, two samples of 2mL of whole blood were collected, respecting the 2-hour […]
Keywords: Child; Microbial sensitivity tests; Pharmacokinetics; Pharmacologic actions; Vancomycin