einstein (São Paulo). 19/Aug/2025;23:eAO1535.
Seizure treatment in children: can intranasal midazolam be used without an atomizer?
DOI: 10.31744/einstein_journal/2025AO1535
Highlights
■ Intranasal midazolam with an atomizer is a treatment for pediatric status epilepticus.
■ In Brazil, the atomizer is often replaced with a syringe owing to the cost of the device.
■ We compared midazolam plasma levels with and without an atomizer in children with epilepsy.
■ No significant difference was found, supporting the syringe-only use in emergencies.
ABSTRACT
Objective:
Convulsive status epilepticus is the most common neurological emergency in children with high morbidity and mortality rates. Intranasal midazolam administration using an atomizer is the preferred treatment method. However, Brazilian pediatric emergencies often require rapid midazolam instillation using syringes. We aimed to compare the plasma midazolam levels in children who received intranasal medication with an atomizer and those who received medication via rapid instillation using only a syringe to treat seizures.
Methods:
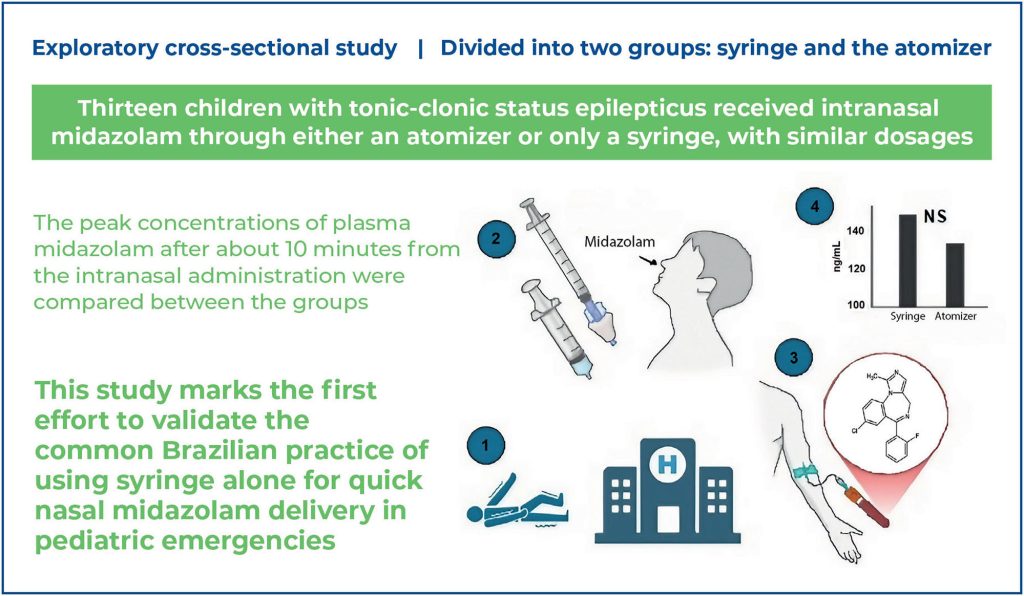
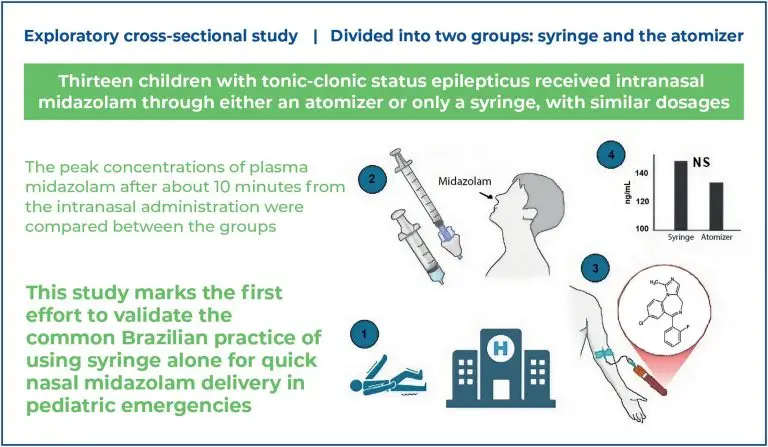
This exploratory cross-sectional study was conducted between January 2022 and March 2024 at the emergency department of a hospital in Southern Brazil. Thirteen children with tonic-clonic status epilepticus received intranasal midazolam via either an atomizer or syringe, at similar dosages. The peak plasma midazolam concentrations, measured approximately 10 min following intranasal administration, were compared between the groups.
Results:
No significant difference was observed in the plasma midazolam concentrations (ng/mL) between the syringe and atomizer groups (median and interquartile range: 148.8 [126.3-293.7] ng/mL versus 133.3 [76.4-176.3] ng/mL; p=0.414), as well as in the time from administration to sampling (8.0 [7.75-10.0] min versus 9.5 [6.75-10.0] min; p=0.710) or in weight-adjusted dose (0.48 [0.44-0.50] mg/kg versus 0.49 [0.47-0.56] mg/kg; p=0.414).
Conclusion:
This study is the first to validate the common Brazilian practice of using syringes alone for rapid nasal midazolam delivery during pediatric emergencies. Future studies should elucidate the clinical outcomes of this approach.
[…]
Keywords: Seizures; Status epilepticus; Administration, intranasal; Midazolam
129