08/Aug/2025
Cost-benefit analysis of implementing Vendor Neutral Archive (VNA) technology in the Brazilian Public Health System
einstein (São Paulo). 08/Aug/2025;23:eGS1219.
View Article08/Aug/2025
Cost-benefit analysis of implementing Vendor Neutral Archive (VNA) technology in the Brazilian Public Health System
DOI: 10.31744/einstein_journal/2025GS1219
Highlights ■ Vendor Neutral Archive implementation has an return on investment of 5.63% in the first year and 10.12% after three years. ■ At least 11,500 exams are needed annually for a positive return on investment in the first year after implementation. ■ Vendor Neutral Archive enhances interoperability and efficiency by standardizing and centralizing image storage. ■ Scalability is key: higher adoption of Vendor Neutral Archive leads to greater financial and operational benefits. ABSTRACT Objective: To conduct an ex-ante cost-benefit […]
Keywords: Brazil; Cloud computing; Cost-benefit analysis; Diagnostic imaging; Government programs; Information storage and retrieval; Public health; Radiology Information Systems; System integration; Vendor neutral archive
02/Jul/2025
Use of propensity score matching to reduce bias in economic evaluations in the context of Brazilian liver transplantation
DOI: 10.31744/einstein_journal/2025AO1329
Highlights ■ Matching methodologies may reduce bias in economic evaluations. ■ Liver transplantation within the Proadi-SUS context has an incremental cost-effectiveness ratio below the Health Technology Incorporation in the Brazilian Health System threshold. ■ The patients who received transplant had a median survival of 10.1 years after list enrollment. ■ The mean cost per transplanted patient was US$ PPP 162,821.96. ABSTRACT Objective: To estimate the incremental cost and survival of patients on the liver transplantation waiting list who were referred […]
Keywords: Brazil; Cost-benefit analysis; Hospital costs; Liver transplantation; Propensity score; Survival rate; Unified Health System; Waiting lists
02/May/2022
Cost-effectiveness analysis of Ado-trastuzumab emtansine for the treatment of residual invasive HER2-positive breast cancer
einstein (São Paulo). 02/May/2022;20:eGS6655.
View Article02/May/2022
Cost-effectiveness analysis of Ado-trastuzumab emtansine for the treatment of residual invasive HER2-positive breast cancer
DOI: 10.31744/einstein_journal/2022GS6655
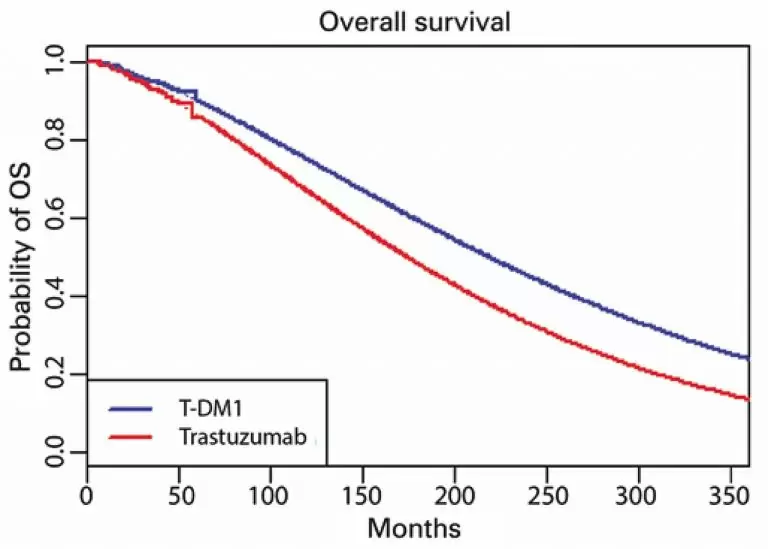
ABSTRACT Objective Human epidermal growth factor receptor 2 (HER2) overexpression occurs in up to 30% of breast cancer cases. Ado-trastuzumab emtansine (T-DM1) is approved to treat residual HER2-positive breast cancer after neoadjuvant therapy. The aim of this study was to determine the quality-adjusted time with symptoms or toxicity and without symptoms or toxicity (Q-TWiST) of T-DM1 compared to trastuzumab for residual invasive HER2-positive breast cancer. Methods The authors developed an analytical model extracting individual patient data and estimated invasive disease-free […]
Keywords: Ado-trastuzumab emtansine; Breast neoplasms; Cost-benefit analysis; HER2-positive; Neoplasm, residual
08/Jul/2021
Comparison of outcomes and costs of surgery versus sclerotherapy to treat hydrocele
DOI: 10.31744/einstein_journal/2021GS5920
ABSTRACT Objective: To evaluate the outcomes and costs associated with surgery versus sclerotherapy as treatment of hydroceles. Methods: A total of 53 men consecutively treated for hydrocele at our organization, between December 2015 and June 2019, were retrospectively analyzed (39 with Jaboulay technique and 14 with sclerotherapy). All charts were reviewed, assessing clinical data, ultrasound findings, surgical data, and post-procedure outcomes. The hospital finance department calculated the cost of outpatient evaluation, complementary tests, supplies, drugs, and professionals’ costs throughout all […]
Keywords: Cost and cost analysis; Cost-benefit analysis; Sclerotherapy; Testicular hydrocele
25/Feb/2019
Cost-effectiveness analysis of abiraterone, docetaxel or placebo plus androgen deprivation therapy for hormone-sensitive advanced prostate cancer
einstein (São Paulo). 25/Feb/2019;17(2):eGS4414.
View Article25/Feb/2019
Cost-effectiveness analysis of abiraterone, docetaxel or placebo plus androgen deprivation therapy for hormone-sensitive advanced prostate cancer
DOI: 10.31744/einstein_journal/2019GS4414
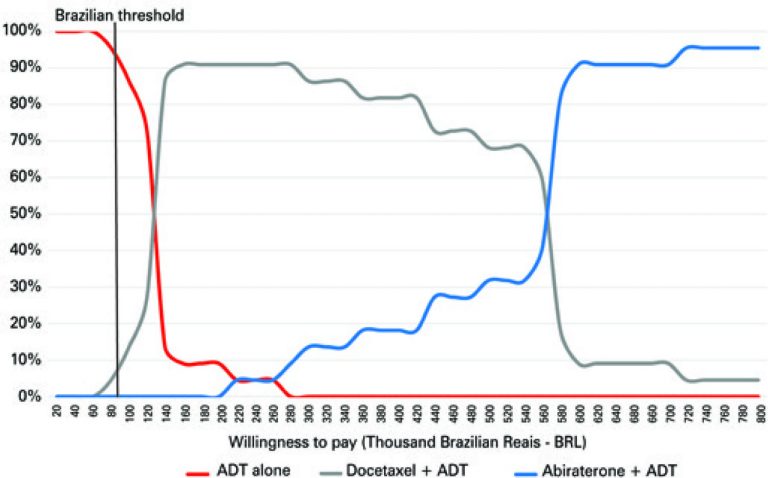
ABSTRACT Objective To evaluate the cost-effectiveness of the addition of chemotherapy or abiraterone to androgen deprivation. Methods We developed an analytical model to determine the cost-effectiveness of the addition of docetaxel or abiraterone versus androgen deprivation therapy alone. Direct and indirect costs were included in the model. The effects were expressed in Quality-Adjusted Life Years adjusted for side effects. Results Compared to androgen deprivation therapy alone, the addition of chemotherapy and of abiraterone generated 0.492 and 0.999, respectively, in Quality-Adjusted […]
Keywords: Cost-benefit analysis; Drug costs; Drug therapy/economy; Hormone therapy/economy; Placebo; Prostatic neoplasms/drug therapy; Public health