einstein (São Paulo). 06/Jun/2024;22:eAO0575.
Prognostic value of programmed cell death ligand 1 (PD-L1) expression in patients with stage III non-small cell lung cancer under different treatment types: a retrospective study
DOI: 10.31744/einstein_journal/2024AO0575
Highlights
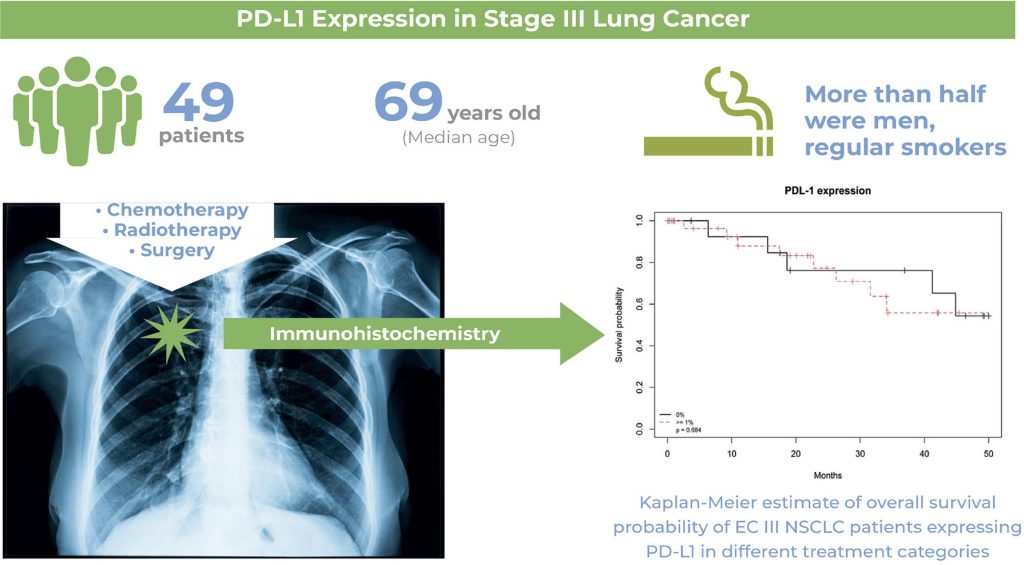
PD-L1 expression by immunohistochemistry is positive in more than half on stage III non-small lung cancer.
PD-L1 expression by immunohistochemistry had no relationship with clinical characteristics.
PD-L1 expression by immunohistochemistry is not related to progression-free or overall survival in patients treated with chemoradiotherapy with or without surgery.
ABSTRACT
Objective:
Currently programmed cell death protein 1 (PD-1) inhibitors in combination with other therapies are being evaluated to determine their efficacy in cancer treatment. However, the effect of PD-ligand (L) 1 expression on disease outcomes in stage III (EC III) non-small cell lung cancer is not completely understood. Therefore, this study aimed to assess the influence of PD-L1 expression on the outcomes of EC III non-small cell lung cancer.
Methods:
This study was conducted on patients diagnosed with EC III non-small cell lung cancer who underwent treatment at a tertiary care hospital. PD-L1 expression was determined using immunohistochemical staining, all patients expressed PD-L1. Survival was estimated using the Kaplan-Meier method. Relationships between variables were assessed using Cox proportional regression models.
Results:
A total of 49 patients (median age=69 years) with EC III nonsmall cell lung cancer and PD-L1 expression were evaluated. More than half of the patients were men, and most were regular smokers. The patients were treated with neoadjuvant chemotherapy, surgery, or sequential or combined chemotherapy and radiotherapy. The median progression-free survival of the entire cohort was 14.2 months, and the median overall survival was 20 months. There was no significant association between PD-L1 expression and disease progression, clinical characteristics, or overall survival.
Conclusions:
PD-L1 expression was not correlated with EC III non-small cell lung cancer outcomes. Whether these findings differ from the association with immune checkpoint inhibitors remains to be addressed in future studies.
[…]
379