einstein (São Paulo). 25/Nov/2025;23:eAE1552.
Implementation of positron emission tomography radiopharmaceuticals labeled with Gallium-68 in a hospital radiopharmacy: experience with more than 7,500 patients
DOI: 10.31744/einstein_journal/2025AE1552
Highlights
■ In-house 68Ga radiopharmaceutical synthesis under GMP in a tertiary hospital radiopharmacy.
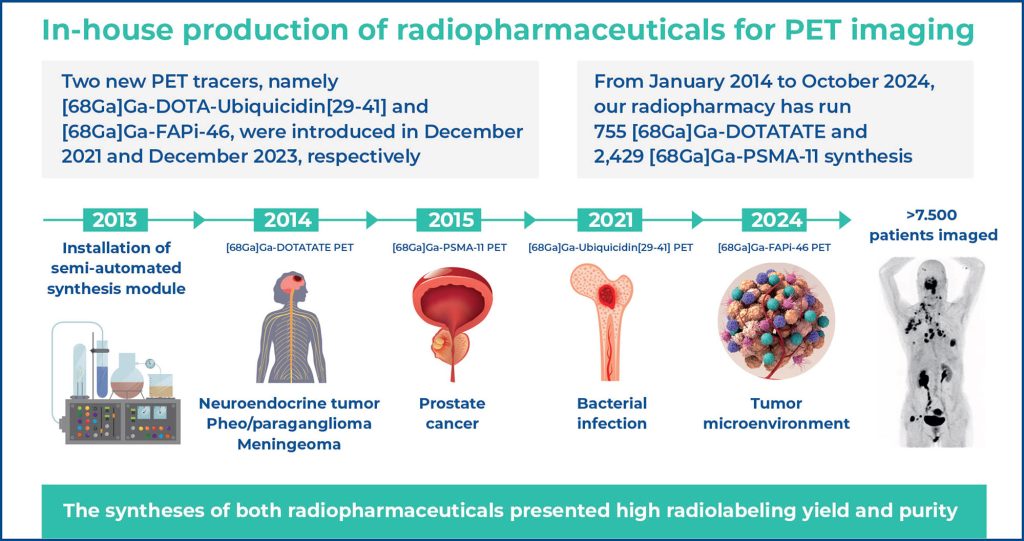
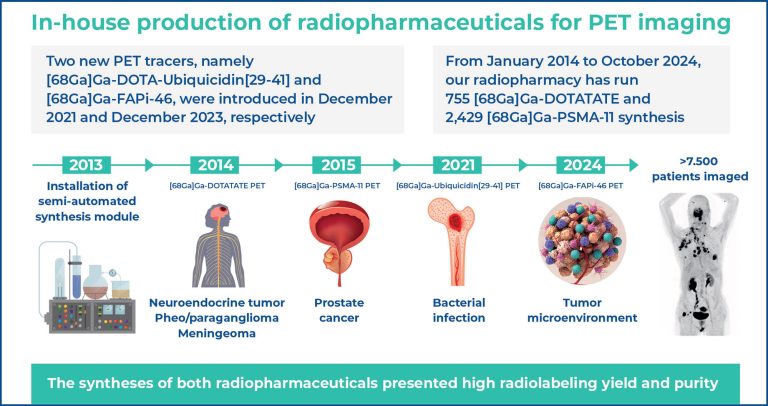
■ Over 7,500 patients underwent imaging with [68Ga]Ga- DOTATATE and [68Ga]Ga-PSMA-11.
■ This is the first radiopharmacy in Brazil to introduce [68Ga] Ga-DOTA-UBI, and [68Ga]Ga-FAPi-46.
ABSTRACT
In this study, we report the implementation of in-house synthesis of gallium-68 (68Ga)-labeled radiopharmaceuticals for positron emission tomography (PET) at a tertiary hospital radiopharmacy. [68Ga]Ga-DOTATATE and [68Ga]Ga-PSMA-11 were introduced into clinical practice in January 2014 and October 2015, respectively, following the establishment of routine automatic module synthesis under good manufacturing practice (GMP) and a certified 68Ge/68Ga generator. Quality control tests, including radiochemical yield, purity, and pyrogenicity, demonstrated high labeling efficiency, excellent purity, and absence of pyrogens. Between January 2014 and October 2024, our radiopharmacy performed 755 [68Ga]Ga-DOTATATE and 2,429 [68Ga]Ga-PSMA-11 syntheses, enabling PET scans for 1,636 and 5,892 patients, primarily for the evaluation of neuroendocrine tumors and prostate cancer, respectively. Two new PET tracers, [68Ga]Ga-DOTA-Ubiquicidin[29-41] (for infection imaging) and [68Ga]Ga-FAPi-46 (targeting cancer-associated fibroblasts), were introduced in December 2021 and December 2023, respectively. Both demonstrated high radiolabeling yields and purities. After preclinical studies and ethics approval, PET scans using these tracers produced high-quality images with favorable preliminary clinical outcomes. To the best of our knowledge, our institution is among the first in Brazil to achieve in-house production and clinical application of these two novel tracers. Over the past decade, the automated GMP-compliant system has consistently enabled reliable synthesis of 68Ga-labeled radiopharmaceuticals, ensuring high radiochemical yield, purity, and high-quality PET imaging, thereby expanding clinical practice with safe and effective new agents.
[…]
147