30/Oct/2018
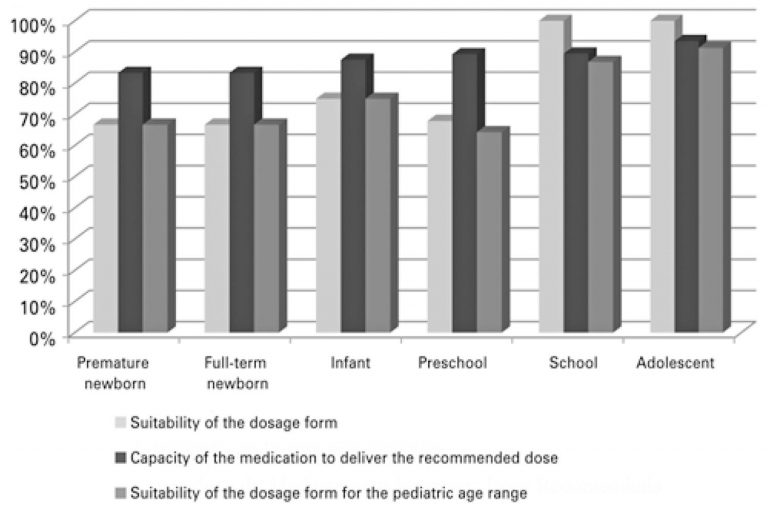
Suitability of new drugs registered in Brazil from 2003 to 2013 for pediatric age groups
einstein (São Paulo). 30/Oct/2018;16(4):eAO4354.
View Article30/Oct/2018
Suitability of new drugs registered in Brazil from 2003 to 2013 for pediatric age groups
DOI: 10.31744/einstein_journal/2018AO4354
ABSTRACT Objective To analyze suitability of new drugs registered in Brazil from 2003 to 2013 for pediatric age groups. Methods A descriptive study of drugs with pediatric indication included in a retrospective cohort of new drugs registered in Brazil. The evaluation of drug suitability for the pediatric age group was performed using the following criteria: suitability of dosage form and capacity to deliver the recommended dose. The drugs were considered adequate for the pediatric age groups when they met both […]
Keywords: Child; Dosage forms; Drug approval; Drug therapy; Reference drugs
01/Jul/2014
Knowledge, perceptions and use of generic drugs: a cross sectional study
DOI: 10.1590/S1679-45082014AO3125
Objective To assess the level of knowledge, perceptions and usage profile for generic drugs among laypersons. Methods A cross-sectional study was conducted with 278 volunteers (180 women and 98 men, aged 37.1±15.8 years). A questionnaire was drawn up with questions on their use, perceptions and knowledge of generic drugs. Results Most respondents (99.6%) knew that generic drugs exist, but only 48.6% were able to define them correctly, while 78.8% of the respondents had some information about generics. This information was […]
Keywords: Drug use; Generic drugs; Patient education; Public policies; Reference drugs
01/Apr/2010
Financial incentives for generic drugs: case study on a reimbursement program
DOI: 10.1590/S1679-45082010AO1478
ABSTRACT Objective: To discuss the use of financial incentives in choice of medication and to assess the economic results concerning the use of financial incentives to promote the use of genetic medication in lieu of reference drugs in a company with a reimbursement program. Methods: A case study was carried out in a large supermarket. The data was obtained in the company responsible for managing medication. The study reached 83,625 users between August 2005 and July 2007. The data was […]
Keywords: Drugs, generic/supply & distribution; Health management; Reference drugs; Reimbursement, incentive/economics