2 results
10/Mar/2025
10/Mar/2025
DOI: 10.31744/einstein_journal/2025AO0964
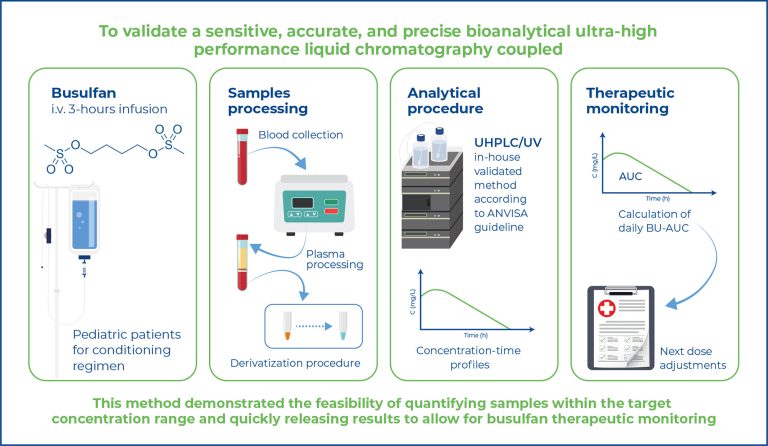
Highlights ■ We validated the UHPLC/UV method for accurate busulfan quantification in plasma. ■ Inaccuracy and imprecision were below 15%, ensuring reliable therapeutic drug monitoring results. ■ This enables effective pharmacokinetic studies with rapid turnaround times in patient samples. ABSTRACT Objective: This study aimed to validate a sensitive, accurate, and precise bioanalytical ultrahigh- performance liquid chromatography coupled with ultraviolet (UHPLC/UV) method for the determination of busulfan in human plasma using 1,6-bis-(methanesulfonyloxy) hexane as an internal standard for therapeutic drug monitoring. […]
Keywords: Busulfan; Calibration; Chromatography, high pressure liquid; Drug monitoring; Hematopoietic stem cell transplantation; Hospital, public; Pharmacokinetics
01/Apr/2010
01/Apr/2010
DOI: 10.1590/S1679-45082010AO1499
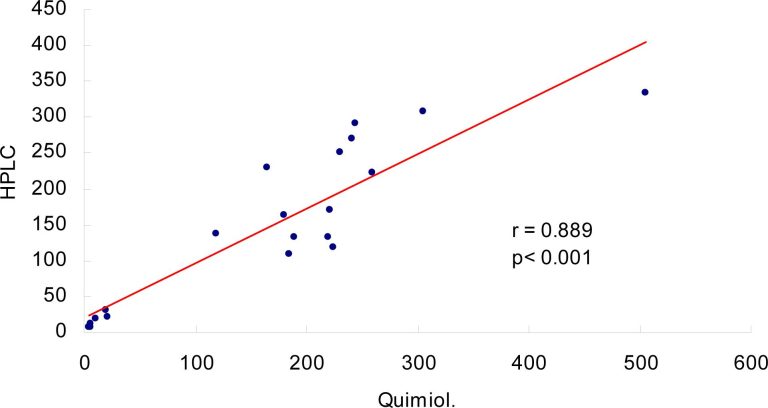
ABSTRACT Objective: To compare the results for homocysteine concentration using chemiluminescence and HPLC methods in samples from school-age children. In addition, to determine the reference values for patients of this age group and assess the real prognostic value of homocysteine in healthy children. Methods: A prospective observational study was undertaken to determine plasma levels of homocysteine using two different assays, HPLC and chemiluminescence, in 185 samples from school-age children living in Santo Andre, with no chronic or inflammatory diseases, and […]
Keywords: Chemiluminescences measurements; Chromatography, high pressure liquid; Homocysteine