einstein (São Paulo). 23/Jul/2025;23:eAO1665.
Translation and validation of the Brazilian Portuguese version of the quality of life vascular access device questionnaire for chemotherapy patients
DOI: 10.31744/einstein_journal/2025AO1665
Highlights
■ Assessing how vascular access devices affect quality of life is essential to effectively manage chemotherapy patients.
■ The translation and validation of the QoLVAD questionnaire demonstrated that the Brazilian Portuguese version can be applied to the Brazilian population.
ABSTRACT
Background:
The use of long-term devices for chemotherapy in neoplastic diseases is very common. The CAVA trial was an extensive study that prospectively evaluated more than 1000 patients undergoing chemotherapy and randomly allocated them to three groups based on the type of catheters used: peripherally inserted central catheters, Port, or Hickmann. For this study, the Quality of Life Vascular Access Device (QoLVAD) questionnaire was administered to assess the impact of these devices (abovementioned catheters) on the patients’ daily lives. routine However, no Brazilian Portuguese version of the questionnaire exists, hindering its use among Brazilian patients.
Objective:
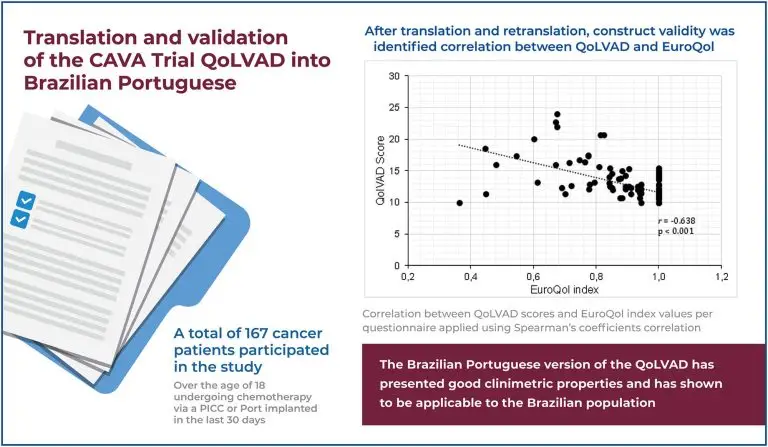
To translate the CAVA trial QoLVAD to Brazilian Portuguese and validate it so that it can be used in the Brazilian population. Methods: A total of 167 patients with long term vascular access devices for chemotherapy participated in the study. After translation and retranslation, construct validity was analyzed by identifying the correlation between QoLVAD and the European Quality of Life Questionnaire (EuroQol). To determine the reliability, internal consistency and test-retest analysis with at least a 7-day interval between two administrations of the questionnaire were calculated.
Results:
The results revealed good internal and external consistency of the QoLVAD. Significant correlations were found between the QoLVAD and EuroQol (r -0,658 and p<0.001).
Conclusion:
The Brazilian Portuguese version of the QoLVAD exhibited good clinimetric properties and proved to be applicable to the Brazilian population.
[…]
61