30/Oct/2018
Suitability of new drugs registered in Brazil from 2003 to 2013 for pediatric age groups
einstein (São Paulo). 30/Oct/2018;16(4):eAO4354.
View Article30/Oct/2018
Suitability of new drugs registered in Brazil from 2003 to 2013 for pediatric age groups
DOI: 10.31744/einstein_journal/2018AO4354
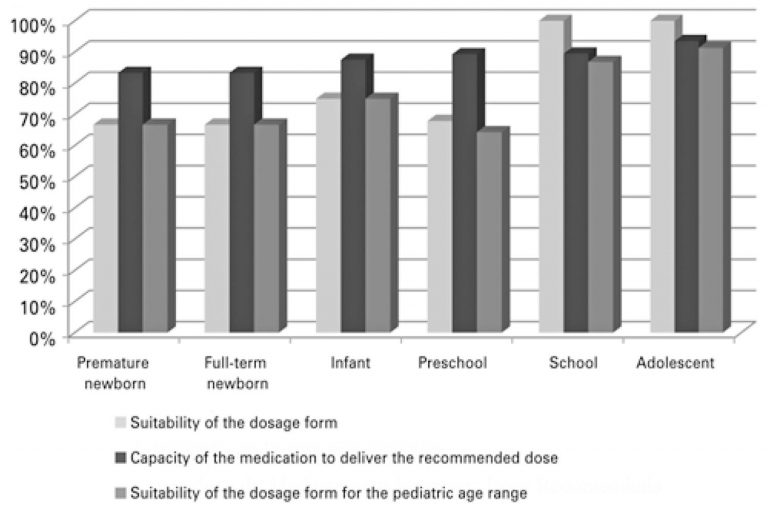
ABSTRACT Objective To analyze suitability of new drugs registered in Brazil from 2003 to 2013 for pediatric age groups. Methods A descriptive study of drugs with pediatric indication included in a retrospective cohort of new drugs registered in Brazil. The evaluation of drug suitability for the pediatric age group was performed using the following criteria: suitability of dosage form and capacity to deliver the recommended dose. The drugs were considered adequate for the pediatric age groups when they met both […]
Keywords: Child; Dosage forms; Drug approval; Drug therapy; Reference drugs