einstein (São Paulo). 10/mar/2025;23:eAO0964.
Validation of an ultra-high performance liquid chromatography/UV method to quantify busulfan in plasma: application to therapeutic drug monitoring
DOI: 10.31744/einstein_journal/2025AO0964
Highlights
■ We validated the UHPLC/UV method for accurate busulfan quantification in plasma.
■ Inaccuracy and imprecision were below 15%, ensuring reliable therapeutic drug monitoring results.
■ This enables effective pharmacokinetic studies with rapid turnaround times in patient samples.
ABSTRACT
Objective:
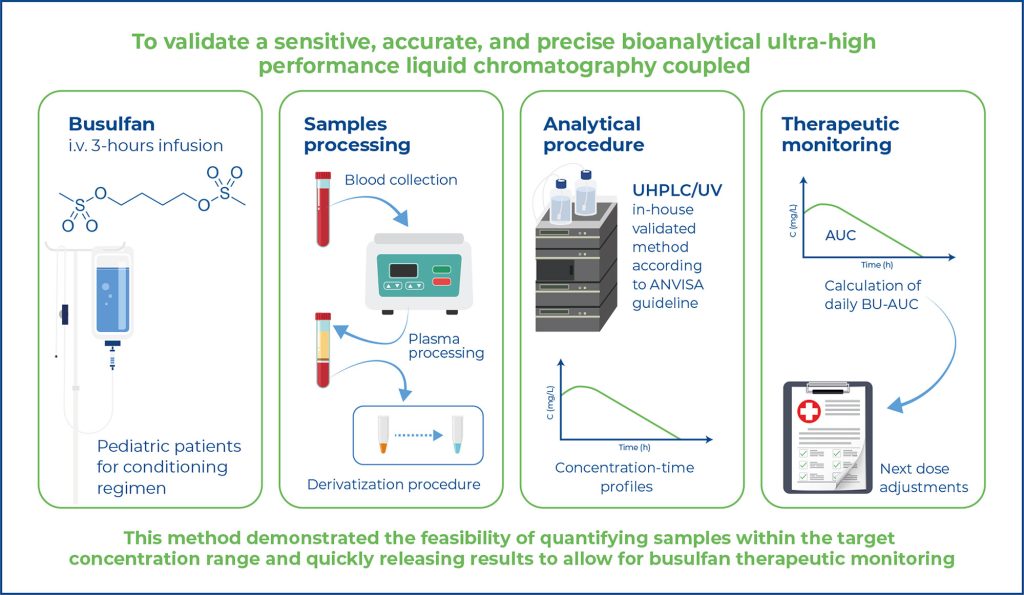
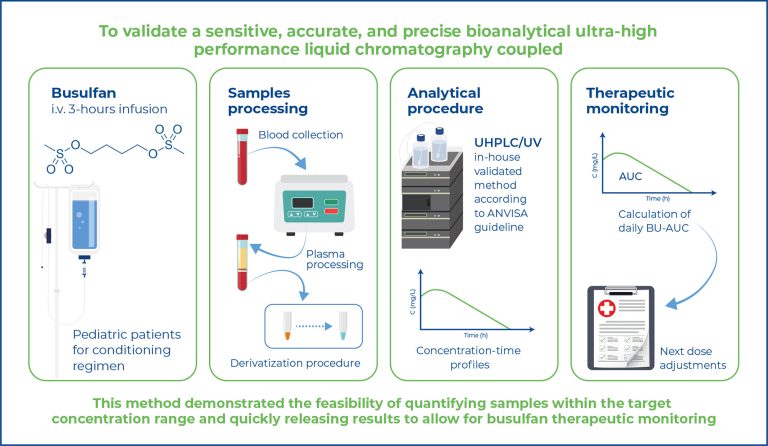
This study aimed to validate a sensitive, accurate, and precise bioanalytical ultrahigh- performance liquid chromatography coupled with ultraviolet (UHPLC/UV) method for the determination of busulfan in human plasma using 1,6-bis-(methanesulfonyloxy) hexane as an internal standard for therapeutic drug monitoring.
Methods:
Plasma samples were deproteinized with acetonitrile (1:2, v/v) and, after derivatization with sodium diethyl dithiocarbamate, submitted to liquid-liquid extraction with ethyl acetate and evaporation at 50ºC under a nitrogen stream. Analyses were performed on a Shimadzu® system using a C18 column and isocratic elution with methanol/water (70:30, v/v) at a flow rate of 0.4mL min-1 and detection at 277nm.
Results:
The retention times of busulfan and the IS were approximately 2.87 and 6.35 min, respectively. The plasma calibration curves were linear in the concentration range of 0.5-10 μg mL-1 with a coefficient of determination greater than 0.99. The lower limit of quantification was 0.5 μg mL-1. The inaccuracies and imprecisions of this method are less than 15%. The applicability of this method to pharmacokinetic studies was confirmed using patient samples obtained after 4 h of 3.2- 5.4 mg kg-1 busulfan intermittent infusion.
Conclusion:
This method demonstrated the feasibility of quantifying samples within the target concentration range and quickly releasing results to allow for busulfan therapeutic monitoring.
[…]
252