einstein (São Paulo). 12/ago/2024;22:eAO0588.
Comparison of the effects of high-flow nasal cannula and bilevel positive airway pressure treatments as respiratory physiotherapy interventions for children with asthma exacerbation: a randomized clinical trial
DOI: 10.31744/einstein_journal/2024AO0588
Highlights
Positive airway pressure may improve the severity of asthma exacerbation.
A high-flow nasal cannula can improve respiratory physiotherapy outcomes.
Positive airway pressure is indicated for moderate asthma exacerbation.
Bilevel positive airway pressure and high-flow nasal cannula use may improve the pulmonary function.
ABSTRACT
Objective:
To evaluate and compare the efficacy of high-flow nasal cannula treatment and that of bilevel positive airway pressure treatment as respiratory physiotherapy interventions for pediatric patients who are hospitalized because of asthma exacerbation.
Methods:
During a randomized clinical trial, treatment was performed using a high-flow nasal cannula and bilevel positive airway pressure for hospitalized children with asthma. After randomization, data regarding lung function, vital signs, and severity scores (pulmonary index, pediatric asthma severity, and pediatric asthma scores) were collected.
Results:
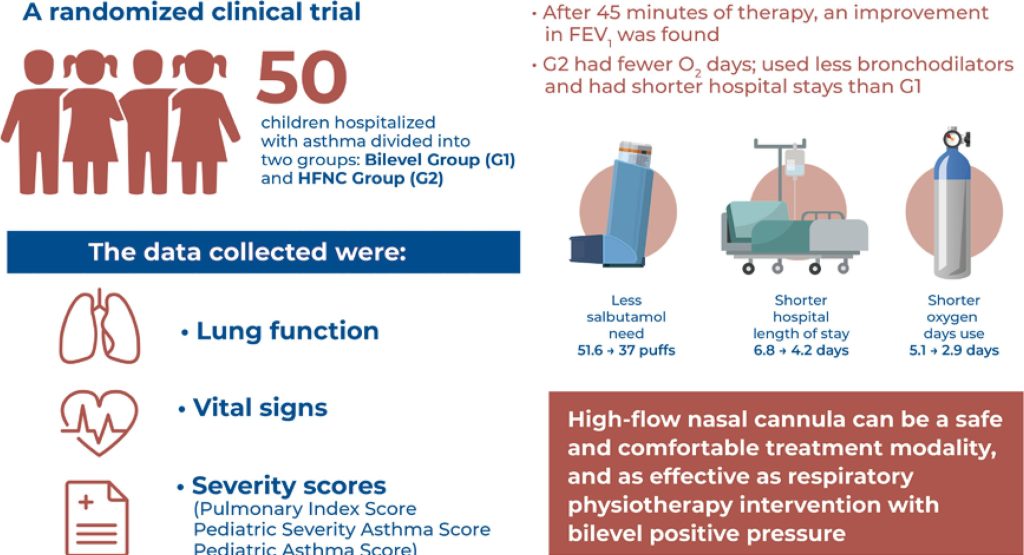
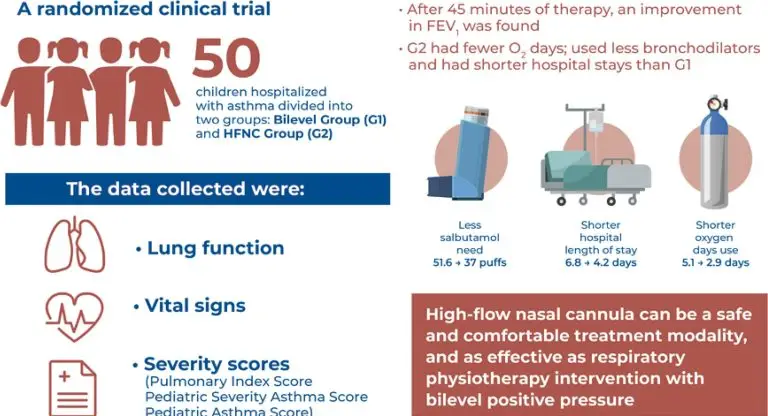
Fifty patients were included in this study (25 in the Bilevel Group and 25 in the high-flow nasal cannula group). After 45 minutes of therapy, an improvement in the forced expiratory volume in 1 second was observed. The high-flow nasal cannula group required fewer days of oxygen (O2) use, used fewer bronchodilators (number of salbutamol puffs), and required shorter hospitalization periods than the Bilevel Group (6.1±1.9 versus 4.3±1.3 days; 95% confidence interval, -5.0 to -0.6)
Conclusion:
A high-flow nasal cannula is a viable option for the treatment of asthma exacerbation because it can reduce the hospitalization period and the need for O2 and bronchodilators. Additionally, it is a safe and comfortable treatment modality that is as effective as bilevel positive airway pressure.
ClinicalTrials.gov Identifier: NCT04033666.
[…]
536