einstein (São Paulo). 18/nov/2024;22:eAO0839.
Severe murine schistosomiasis results from disrupted CD4+ T-cell modulation by immunodominance of a single egg epitope
DOI: 10.31744/einstein_journal/2024AO0839
Highlights
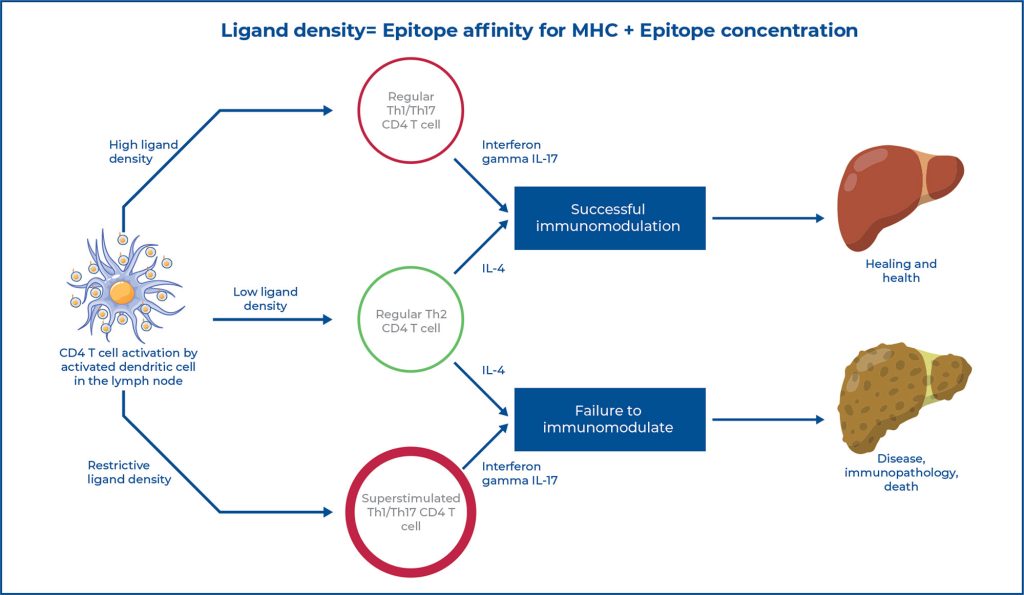
■ Restrictive ligand density can be pathological.
■ Restrictive ligand density can be neutralized.
■ Restrictive ligand density neutralization may the used to heal or prevent disease.
ABSTRACT
Objective:
This study examined the correlation between immunodominance of the major egg antigen Sm-p40234-246, a robust Th1/Th17 anti-egg CD4 T-cell response, and severe liver immunopathology in experimental murine schistosomiasis. It serves as a platform to analyze how varying degrees of immunodominance affect CD4+ T cell modulation and disease outcomes.
Methods:
We used a murine model of schistosomiasis to investigate the effects of immunodominance. Infected mice were divided into two groups: one treated with a combination of epitopes targeting immunodominance of the major egg antigen Sm-p40 and the other with a mock mixture of non-immunogenic epitopes. Liver granuloma area, a hallmark of schistosomiasis pathology, was quantified using histological and morphometric analyses. The average granuloma areas between the treated and untreated groups were compared using one-way ANOVA with Tukey’s multiple comparison test. Additionally, we isolated CD4+ T cells from mesenteric lymph nodes, stimulated them with specific egg antigens, and collected purified supernatants to assess their signature cytokine secretion profiles for each treatment group.
Results:
Results showed that strong immunodominance of a single egg epitope undermines effective CD4+ T-cell modulation, promoting a strongly polarized Th1/Th17 pathogenic response. Conversely, neutralizing this immunodominance produces the opposite restorative effect.
Conclusion:
Immunodominance is an important pathogenic component that influences CD4+ T cell modulation in experimental murine schistosomiasis. Moreover, immunodominance can be used to treat these and other important CD4+ T cell-mediated diseases.
[…]
238